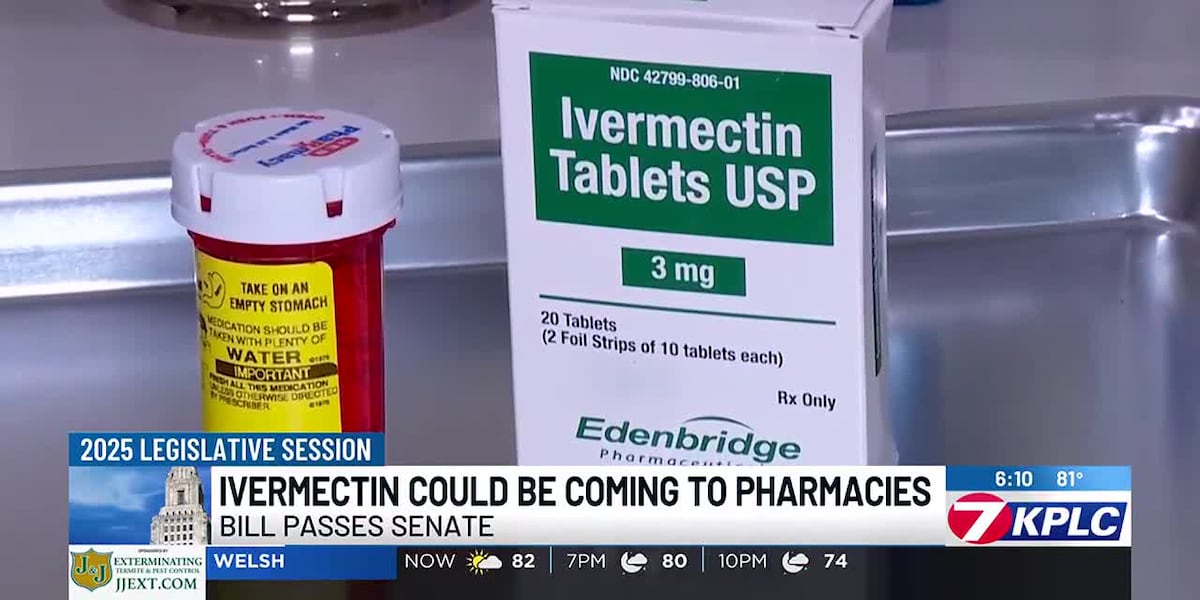
State Legislatures Debate Ivermectin Access Amidst Conflicting Medical Opinions
Legislatures in states like Louisiana and New Hampshire are debating bills that would allow easier access to Ivermectin, an anti-parasitic drug, particularly for treating COVID-19. These efforts are fueled by some lawmakers who believe the drug was unfairly restricted during the pandemic. However, medical professionals and other lawmakers raise concerns about its efficacy and the lack of FDA approval for COVID-19 treatment.
 kplctv.com
kplctv.com
 WMUR
WMUR
 Shreveport Times
Shreveport Times
 UnionLeader.com
UnionLeader.com
Online Pharmacies: Man Dies After Overdose, Counterfeit Risks, and Warnings
This article addresses the critical issue of illegal online pharmacies, highlighting the dangers of purchasing prescription drugs from unregulated sources. It details the accidental overdose death of a man who obtained medication online, discusses the prevalence of counterfeit drugs, and provides guidance on identifying legitimate online pharmacies and safe practices. The article emphasizes the need for increased vigilance and regulatory action.

 WJAR
WJAR
 The Irish Sun
The Irish Sun
 Yahoo News UK
Yahoo News UK
 Chemist+Druggist
Chemist+Druggist
Man Dies After Overdosing on Drugs Bought from Illegal Online Pharmacies
A 45-year-old man in Wales died from an accidental overdose after buying prescription medication from illicit online pharmacies. The case highlights the risks associated with unregulated online pharmacies, including the lack of proper medical oversight, potential for counterfeit drugs, and ease of access for vulnerable individuals seeking prescription medication. Experts warn about the prevalence of illegal online pharmacies and the dangers of purchasing drugs from them.

 WJAR
WJAR
 The Irish Sun
The Irish Sun
 Yahoo News UK
Yahoo News UK
 Chemist+Druggist
Chemist+Druggist
Delhi HC Slams Ramdev for New Video Targeting Rooh Afza, Considers Contempt
The Delhi High Court has reprimanded Baba Ramdev for releasing a new video allegedly containing disparaging remarks against Hamdard's Rooh Afza, despite a previous undertaking. The court is considering a contempt notice and has ordered Ramdev to remove the offensive portion of the video. The legal battle stems from Ramdev's earlier 'sharbat jihad' comments.

 The Tribune
The Tribune
 NDTV
NDTV
 The Indian Express
The Indian Express
 The Hindu
The Hindu
Ramdev Faces Court Scrutiny Over Disparaging Remarks and Misleading Ads
Ramdev, the yoga guru and entrepreneur, is embroiled in legal battles over disparaging remarks about competitors like Hamdard's Rooh Afza and misleading advertisements. The Delhi High Court has repeatedly reprimanded him for violating court orders and making controversial statements. Patanjali, his company, faces scrutiny for its marketing practices and product claims.

 The Tribune
The Tribune
 NDTV
NDTV
 Hindustan Times
Hindustan Times
 Times of India
Times of India
Delhi HC Orders Ramdev to Remove 'Offensive' Video Against Rooh Afza
The Delhi High Court has directed Baba Ramdev to remove an 'offensive' video targeting Hamdard's Rooh Afza following accusations of contempt of court. This order comes after Ramdev allegedly violated a previous gag order by posting a new video containing disparaging remarks about the herbal drink. The court criticized Ramdev for disregarding its directives, leading to a warning of contempt action and a subsequent pledge from Ramdev to delete the contentious content.

 The Tribune
The Tribune
 NDTV
NDTV
 Times of India
Times of India
 Inshorts
Inshorts
Ramdev Faces Court Scrutiny Over 'Sharbat Jihad' Remarks and Misleading Ads
Ramdev is facing increased scrutiny from the Delhi High Court regarding his controversial 'sharbat jihad' remarks against Hamdard's Rooh Afza and a history of misleading advertisements. The court has reprimanded Ramdev for flouting orders and making disparaging statements, leading to contempt of court warnings. His business practices and claims about Patanjali products are also under investigation.

 The Tribune
The Tribune
 NDTV
NDTV
 Times of India
Times of India
 The New Indian Express
The New Indian Express
Medetomidine Emerges as Threat in US Illegal Drug Supply, Complicating Opioid Crisis
Reports from the CDC and investigations in multiple cities including Philadelphia, Chicago and Pittsburgh indicate the emergence of medetomidine, an animal sedative, in the illegal drug supply. Medetomidine, often mixed with fentanyl, is causing severe withdrawal symptoms that are resistant to traditional treatments and creating challenges for healthcare providers.

 AP News
AP News
 Centers for Disease Control and Prevention | CDC (.gov)
Centers for Disease Control and Prevention | CDC (.gov)
 WYFF
WYFF
 Inquirer.com
Inquirer.com
Animal Sedative Medetomidine Detected in US Illegal Drug Supply, CDC Reports
The CDC reports the increasing presence of medetomidine, an animal sedative, in the US illegal drug supply, primarily mixed with fentanyl. This has led to a surge in overdoses, especially in Chicago, Philadelphia, and Pittsburgh, where naloxone is proving ineffective. Philadelphia saw medetomidine overtake xylazine in opioid samples, causing unusual fentanyl withdrawal symptoms.

 AP News
AP News
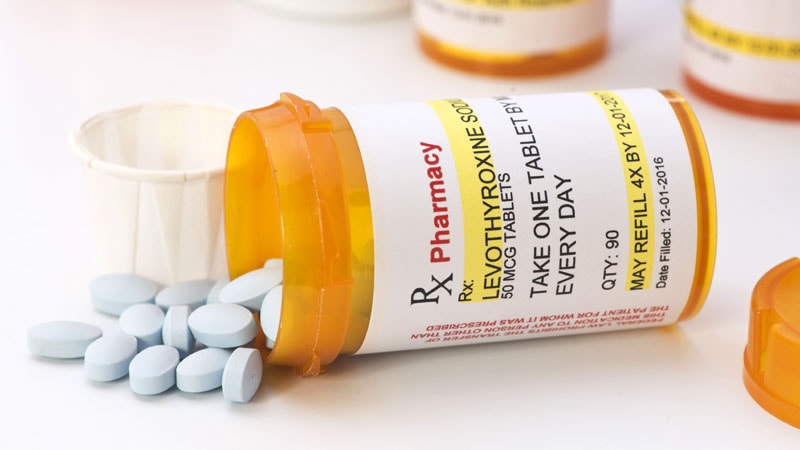
Understanding Increased TSH Levels Despite Levothyroxine Treatment: A Comprehensive Analysis
This article explores the reasons behind increased thyroid-stimulating hormone (TSH) levels in patients taking levothyroxine for hypothyroidism. It identifies common causes such as medication interactions, absorption problems, and underlying malabsorption disorders. Recommendations for levothyroxine use and next steps in management, including medication review and absorption tests, are also discussed.
 Medscape
Medscape
Rising TSH Levels on Levothyroxine: Common Causes and Management Strategies
This article addresses the common clinical problem of elevated TSH levels in patients treated with levothyroxine for hypothyroidism. It identifies medication interference, dietary factors, and malabsorption syndromes as potential causes, and suggests thorough medication reviews, absorption tests, and dietary adjustments as key management steps. The article underscores the importance of patient education and adherence.
 Medscape
Medscape
Elevated TSH on Levothyroxine: Absorption Issues, Medication Interactions, and Management
This article addresses the common clinical problem of rising TSH levels in patients taking levothyroxine for hypothyroidism. It explores causes such as medication interactions (iron, calcium, PPIs), malabsorption disorders, and diet. Recommendations include medication review, absorption tests, and addressing underlying malabsorption issues to optimize levothyroxine effectiveness.
 Medscape
Medscape
FDA Job Cuts: Drug Approval Delays, Safety Concerns, and Industry Impact
Recent US FDA job cuts, stemming from HHS restructuring, are causing drug approval delays, hindering safety monitoring, and prompting concerns about innovation in the biotech sector. The cuts impact drug reviewers, potentially slow down approval processes, and disrupt safety programs. This situation leads to uncertainty and potential risks in the healthcare industry.
 C&EN
C&EN
 BioSpace
BioSpace
 statnews.com
statnews.com
 Global Venturing
Global Venturing
FDA Staff Exodus and Reorganization Disrupt Drug Approval Processes, Safety Monitoring
The FDA is facing significant challenges due to staff departures, proposed reorganization, and budget cuts. This is leading to delays in drug approvals, disruptions in safety monitoring programs, and increased concerns within the biotech industry about the agency's ability to fulfill its critical functions. The situation is further complicated by the perceived 'revolving door' between the FDA and pharmaceutical companies.
 C&EN
C&EN
 BioSpace
BioSpace
 statnews.com
statnews.com
 Global Venturing
Global Venturing
FDA Job Cuts Impact Drug Safety Monitoring, Endangering Patient Safeguards
Recent FDA layoffs, primarily affecting the Division of Drug Information, are disrupting drug safety monitoring. Terminated staff report a severe reduction in team size, impacting programs like REMS. This leads to healthcare provider confusion regarding prescription restrictions and program statuses, potentially endangering patient safety. The situation raises concerns about the FDA's ability to handle safety issues and drug recalls effectively.
 C&EN
C&EN
 Genetic Engineering and Biotechnology News
Genetic Engineering and Biotechnology News
 Regulatory Affairs Professionals Society | RAPS
Regulatory Affairs Professionals Society | RAPS
 Daily Kos
Daily Kos
FDA Reverses Layoffs After Cuts Disrupt Drug and Food Safety Work
The FDA is reinstating some employees after layoffs led to disruptions in drug and food safety work, including reinstating staff for foreign inspection travel bookings and scientists in drug/food safety labs. Concerns arose from potential fines for missed document deadlines, confusion over the status of drug safety programs, and disruptions to drug safety monitoring.
 C&EN
C&EN
 AP News
AP News
 CBS News
CBS News
 statnews.com
statnews.com
Supreme Court Urges Generic Drug Prescription Mandate to Curb Pharma Bribery
The Supreme Court is examining a petition seeking regulation of unethical marketing practices in the pharmaceutical industry. The court suggested mandating doctors to prescribe generic medicines nationwide, similar to an existing directive in Rajasthan, to tackle alleged bribery and overuse of branded drugs. The case highlights concerns over rising healthcare costs and the influence of pharmaceutical companies.
 NDTV
NDTV
 Verdictum
Verdictum
 The420.in
The420.in
 CNBC TV18
CNBC TV18
Supreme Court Mulls Mandating Generic Drug Prescriptions to Curb Pharma Bribes
The Supreme Court is evaluating whether to mandate doctors to prescribe generic medicines nationwide, following concerns about pharmaceutical companies bribing doctors to promote branded drugs. The court is responding to a petition seeking enforceable guidelines to regulate unethical marketing practices. A similar executive instruction is already in place in Rajasthan. The next hearing is scheduled for July 24.
 NDTV
NDTV
 Verdictum
Verdictum
 The420.in
The420.in
 Law Trend
Law Trend
CVS Health Prioritizes Wegovy, Drops Zepbound from Standard Formulary
CVS Health's Caremark will favor Wegovy over Zepbound in its standard formularies starting July 1, 2025. This decision, driven by price negotiations and strategic contracting, impacts millions of patients, potentially limiting access to Zepbound and increasing out-of-pocket costs. Eli Lilly is responding through direct-to-consumer channels and partnerships. The move highlights the complex dynamics between pharmaceutical companies and pharmacy benefit managers.

 AP News
AP News
 Reuters
Reuters
 CNBC
CNBC
 Forbes
Forbes
CVS Caremark Chooses Wegovy as Preferred Weight-Loss Drug, Drops Zepbound
CVS Caremark, a major pharmacy benefit manager, will favor Novo Nordisk's Wegovy over Eli Lilly's Zepbound on its standard drug formularies starting July 1, 2025. This decision aims to provide a more affordable weight-loss option for patients. CVS will also offer Wegovy at a discounted price for those without insurance and has partnered with Novo Nordisk's direct-to-consumer pharmacy.

 AP News
AP News
 Reuters
Reuters
 PR Newswire
PR Newswire
 CNBC
CNBC